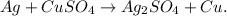Answer:
Zinc (Zn).
We obtain Ag2SO4.
Step-by-step explanation:
We can use another metal that can displace copper ions from solution of copper sulfate CuSO4. The key is to find a metal that contains an oxidation state of +2. If you go to see the periodic table, you can realize that a common example of this is Zn because it only contains +2 as an oxidation state.
Now, if we put a piece of silver into a solution containing Cu (2+), in this case, CuSO4 contains Cu (+) ions, we will obtain silver sulfate: