First, we have that on day 0, the percent of remaining iodine is 100%. This percent reaches 50% on day 8; so the half-life of iodine-131 is 8 days.
With this data, we can find the remaining amount based on the following formula:
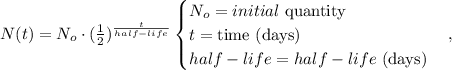
We have that the initial quantity is 50 grams and the time is 32 days. Replacing all data, we obtain:
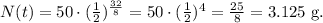
So, the remaining quantity of iodine-131 after 32 days is 3.1 grams.