Answer
449.4 grams
Step-by-step explanation
The balanced chemical equation of the reaction is;
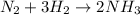
From the balanced chemical equation;
3 moles of H₂ reacted with 1 mole of N₂ to produce 2 moles of NH₃
Molar mass of H₂ = 2.016 g/mol
Molar mass of N₂ = 28.0134 g/mol
Molar mass of NH₃ = 17.031 g/mol
Convert mole to gram using the formula;
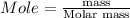
For 1 mole N₂
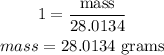
For 3 moles H₂
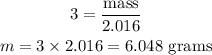
For 2 moles NH₃
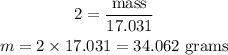
We can now calculate, the mass of NH₃ that can be produced from 79.8 grams of H₂ as follows:
From the balanced equation we can say;
6.048 grams H₂ → 34.062 grams NH₃
∴ 79.8 grams H₂ → x grams NH₃
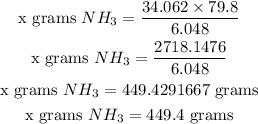
Therefore, 449.4 grams of Ammonia is produced if you started with 79.8 grams of Hydrogen.