These solutions that are made from stock solutions, follow the next steps:
Step 1
Formula used:
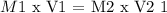
Where,
M1 = concentration of stock solution
V1 = volume of the stock solution
----
M2 = concentration of new solution
V2 = volume of new solution
------------
Step 2
These kinds of solutions are prepared with sulfuric acid and water as a solvent.
Information provided:
M1 = 16.0 M
V1 = unknown
---
M2 = 3.0 M
V2 = 1.5 L
------------
Step 3
Procedure:
From (1),
V1 = M2 x V2/M1
V1 = (3.0 M x 1.5 L)/16.0 M = 0.28 L of stock solution
Answer:
How would you prepare the new solution?
0.28 L of stock solution are taken. Next, 1.22 L of water is added to the 0.28 L to complete 1.5 L of the new solution. Finally, the new solution (1.5 L and 3.0 M) is ready to be used.