To answer this question, we have to use the given balanced equation to state a stoichiometric ratio between the moles of N2 and the moles of H2 that react with each other.
According to this, 1 mole of N2 reacts with 3 moles of H2, use this ratio to find how many moles of H2 react with 7.5 moles of N2:
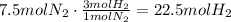
It means that 22.5moles of H2 will be needed to react with 7.5moles of N2.