2N2O5(g) → 4NO2(g) + O2(g) (this is our reaction and it is already balanced)
2 moles N2O5 disappears (reactant)
4 moles NO2 appears (product)
We can work with equations here that represent the disappearance and appearance of compounds:
The disappearance of N2O5: rate of disappearance
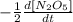
The appearance of NO2: rate of appearance
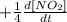
We can do this:
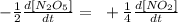
We can do this because the disappearance of reactants involves the appearance of the product.
Then, we clear the rate of N2O5:
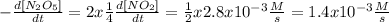
Now the rate of disappearance of N2O5 = 1.4x10^-3 M/s