Answer:
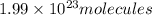
Explanations
Given the following parameters
Mass of P2O5 = 48 grams
Convert mass to moles
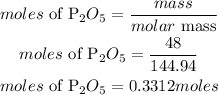
Convert moles to molecules uusing the Avogadros constant. According to the Avogadro's constant;
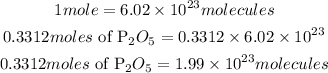
Hence the number of molecules in 48 grams of P2O5 is 1.99 * 10^23 molecules