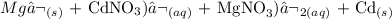Answer:
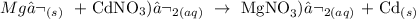
It is a single replacement (SR) reaction also known as displacement reaction
Step-by-step explanation:
Here, we want to write the equation of reaction for the single replacement of magnesium and cadmium (II) nitrate
Magnesium ion is higher up than cadmium (ii) ion in the electrochemical series. What this means is that it can displace it from its salt
We have the equation of reaction in words as:
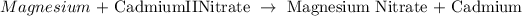
Using their symbols, we have the equation above as: