Step 1
Z = atomic number
A = mass number
n = number of neutrons
The next formula is used:
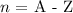
-----------------------
Step 2
Information provided:
Element = Gold => The atomic number (Z) for gold is 79
(Z is found in the periodic table)
n = 118 nutrons
-----------------------
Step 3
From step 1, A is found as follows:
n = A - Z
n + Z = A
118 + 79 = A
197 = A
Answer: The mass number (A) = 197