Answer:
The correctly list for the coefficients (in order left to right) is: 1, 2, 1, 4. (The first option)
Step-by-step explanation:
To choose the correct coeffiecients for the given reaction, it is necessary to balanced que reaction, making sure that there is the same amount of each element in the reactants side and in the products side:
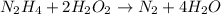
1 mole of N2H4 reacts with 2 moles of H2O2 to produce 1 mole of N2 and 4 moles of H2O.
So, the correctly list for the coefficients (in order left to right) is: 1, 2, 1, 4.