Answer:
92g of CaCO3 are made.
Step-by-step explanation:
From the balanced equation, we know that 1 mole of sodium carbonate produces 1 mole of CaCO3. Since the relation between the reactant and the product is 1:1, 0.920 moles of sodium carbonate will produce 0.920 moles of CaCO3.
With the molar mass of CaCO3 (100g/mol) we can convert 0.920 moles into grams:
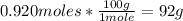
So, 92g of CaCO3 are made.