The question is mostly solved. The definition of heat is used for this problem which tells us:
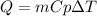
Where,
Q is the heat added to the system, 120 J
m is the mass of the metal, 5.0 g
Cp is the specific heat of the metal, 0.385J/g°C
dT is the change of temperature:
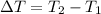
T2 is the final temperature, unknown
T1 is the initial temperature, 22°C
We clear the final temperature from the equation:
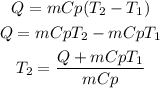
Now, we replace the known data:
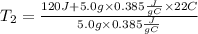
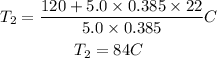
Answer:
The final temperature of the metal will be 84°C
The change in the temperature will be 84°C-22°C=62°C