Answer
a.) The moles of CO that would be produced = 8 moles
b.) The moles of CO that would be produced = 20 moles
c.) The limiting reactant is Carbon
d.) Theoretical yield of CO = 8 moles
Step-by-step explanation
Consider the given reaction: 5C + 2SO₂ → CS₂ + 4CO
a.) If you had 10 mol of Carbon, the moles of CO that would be produced is calculated using the mole ratio from the equation as follows
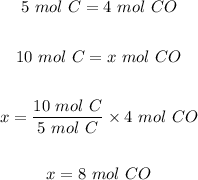
The moles of CO that would be produced = 8 moles
b.) If you had 10 mol of sulfur dioxide, the moles of CO that would be produced is calculated using the mole ratio from the equation as follows
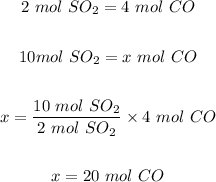
The moles of CO that would be produced = 20 moles
c.) From the equation of reaction; the mole ratio of C to SO₂ is 5:2.
This implies 5 moles of C requires 2 moles of SO₂
If you now had 10 mol of C and SO₂, it implies 10 mol of C will require just 4 mol of SO₂. Therefore, C is the limiting reactant because it will be the first reactant to be completely consumed.
d.) Since the limiting reactant is C (from part c); which determines when the reaction goes to completion and the amount of CO produced, then from part (a), 10 mol C produced 8 mol CO.
Therefore, the theoretical yield of CO = 8 moles