The balanced equation of the combustion of propane is
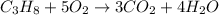
From the above equation, we can say that 1 mole of propane requires 5 moles of oxygen to undergo complete combustion and it produces 3 moles of carbon-di-oxide and 4 moles of water molecules.
Therefore the correct answer is, 'One mole of propane combines with five moles of oxygen to produce four moles of water and three moles of carbon dioxide'.