The balanced reaction for this reaction is:
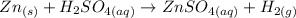
Step 1: We will convert the mass of the Zn metal to moles:

Step 2: We will determine the moles of hydrogen gas that is produced from 0.689813 moles of Zn:
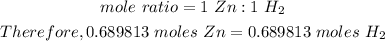
Step 3: In order to determine the volume of gas we use the ideal gas law:
