Working of a calorimeter:
The calorimeter is used to measure the specific heat of substance. The substance is placed inside calorimeter and the starts heating the calorimeter, the heat relased by the calorimeter will be absorbed by the substance present in the calorimeter.
There are following parts in the calorimeter,
Stirrer
Thermometer
Insulation
Crucible
Steel bomb
Ignition wires
Ignition coil
Oxygen supply
When the substance is placed in the calorimeter, then with the help of ignition wires the ignition coil is heated placed in the steel bomb. The heat released by the substance is abosorbed by the calorimeter.
By applying heat balance equation, the specific heat of substance can be calculated. The heat balance equation is given by,
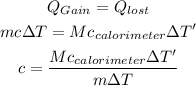
Here, m is the mass of substance, ∆T is the change temperature of the substance, Ccalorimeter is the specific heat of material of calorimeter, ∆T' is the change in temperature of the calorimeter, M is the mass of material of calorimeter.