Given that radioactive substance is reduced by 35 percent in 15 hours.
Let N(0) be the initial quantity and N(15) be the quantity of substance after 15 hours.
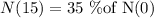
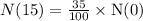
We know that the formula to find the quantity of substance after t hours is
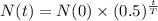
T is the half-life time.
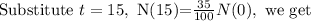
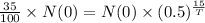
Cancel out N(0), we get
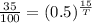
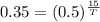
Taking log on both sides, we get
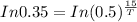
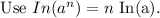
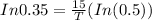
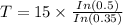
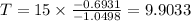
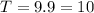
Hence the half-life time is 10 hours.