The ΔH of a reaction can be though of the enthalpy of the products, Hp, minus the enthalpy of the reactants, Hr:
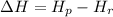
Since energy can't be destroyied or created in a chemical reaction, the energy on the reactant part and on the product part have to be the same.
If no energy is released or absorbed, the enthalpies of products and reactants would be the same. If there are extra energy being release, this means that the reactants had more energy than the products, so it get released.
In enthalpy terms, it means that the enthalpy of the reactants is greater than the enthalpy of the products when energy is released, and this excess is what is released.
If Hr is greater than Hp, the ΔH will be negative.
So, the correct options is "the reaction has a negative "delta H""