Given:
The number of coins, N=31.
The total value of the coins, T=$5.65.
Let x be the number of dimes and y be the number of quarters.
The equation for the number of coins can be expressed as,

We know,
1 dollar=100 cents.
Hence, 1 cent is,
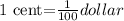
1 quarter=25 cents.
Then, 1 quarter in dollars is,
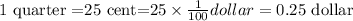
1 dime=10cents.
Then, 1 dime in dollars is,
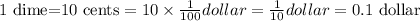
Now, the equation for the total value of coins can be expressed as,
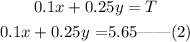
Multiply equation (1) by 0.1.
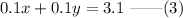
Now, subtract equation (3) from (2) to find the value of y.
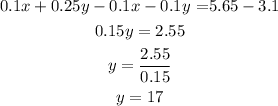
Now, put y=17 in equation (1) to find the value of x.
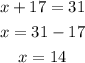
Therefore, the number of dimes is 14 and the number of quarters is 17.