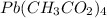In all this table, we can solve on the same steps:
- find out the anion and its charge.
- find out the cation and its charge.
- Combine them so that the total charge is zero.
The acetate is CH₃CO₂⁻, so its charge is 1-. This means that we will use as many acetates as the charge of the cation and we will use only one cation everytime.
Zinc is Zn and its charge is 2+, so it is Zn²⁺, so we will combine it with two acetates:
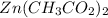
The copper is indicated as copper(I), so its charge is 1+, so Cu⁺, so we combine with 1 acetate:
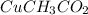
Iron is indicated as iron(III), so its charge is 3+, so Fe³⁺, and we will combine with 3 acetates:
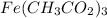
Potassium can only be 1+, so K⁺, and we will combine with 1 acetate:
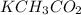
Strontium can only be 2+, so Sr²⁺, and we will combine with 2 acetates:
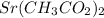
Lead is indicated as lead(IV) so 4+ so Pb⁴⁺, and we will combine with 4 acetates: