To answer this question, we need to order the data in ascending order. Then, we have:
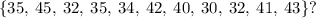
Order data in ascending order:
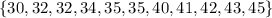
Now to construct the box and whisker plot, we need to find:
• The minimum value for the data (m).
,
• The first quartile (Q1)
,
• The second quartile or the median (Q2 = M)
,
• The third quartile (Q3)
,
• The maximum value (M)
To find all of them, we can proceed as follows:
Minimum
The minimum is the least value in the data set. In this case, we have that minimum is equal to 30. Then, m = 30.
The first quartile (Q1)
This is the value for which 25% of the values are below it, and 75% of the values are above this value. To find it, we need to identify the lower half of the data without taking into account the median.
We have that the data is, and we can see all the important values to graph the box and whisker plot:
To find Q1, we can see that the lower half (without the median) are the next values:
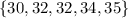
The first quartile is the "median" for these values. The number that divides the set into two parts is 32.
Therefore, the first quartile is 32, Q1 = 32.
The median (or the second quartile)
The value that divides into both equal parts the data set is 35 since we have the same number of values below and above 35, that is, we have 5 numbers below and 5 numbers above 35. In other words, the median is the value for which 50% of the data is above and below this value.
Therefore, median = 35.
The third quartile (Q3)
In this case, we need to identify the upper half of the data set without taking into account the median, and then find the "median" for this data set:
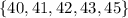
And the "median" for this value is 42.
Therefore, the third quartile is 42. We have that 75% of the values are below it, and 25% of the values are above it.
Therefore, Q3 = 42.
Maximum
The maximum value is the greatest value for the data set. In this case, we have that the maximum is 45.
Therefore, maximum = 45.
Graphing the data as a box and whisker plot
The values we need to graph are:
• Minimum = 30
,
• Q1 = 32
,
• Median = 35
,
• Q3 = 42
,
• Maximum = 45
To graph this data, we need to draw a line as follows:
We need to have a line and draw the corresponding values in an equally spaced number line. We identified the values for the minimum, Q1, the median, Q3, and the maximum. After that, we draw a box with the ends through the points for the first and third quartiles. We join the minimum and maximum values using a segment of a line. The median is represented by a vertical line inside the box as shown above. And we have the box and whisker plot of the given data set.
Finally, we have that the graph is: