Answer;
a) CO2 and H2O
![b)\text{ }2C_4H_(10)+13O_2\operatorname{\rightarrow}8CO_2+10H_2O]()
c) 33.28 grams
Explanations
The combustion of an hydrocarbon will product carbondioxide and water as products.
a) The products are carbon dioxide(CO2) and water (H2O).
b) The balanced form of the reaction is given as shown below;
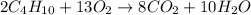
Since all the atoms of elements on both sides are equal, hence the equation is baanced.
c) Given the parameters
Mass of C4H10 = 9.3 grams
Calculate the moles of C4H10
moles of butane = mass/molar mass
moles of butane = 9.3/58.12
moles of butane = 0.16moles
According to stoichiometry, 2moles of butane reacts with 13moles of oxygen, the moles of oxygen required willl be;
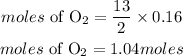
Determine the mass of oxygen
Mass of oxygen = moles * molar mass
Mass of oxygen = 1.04 * 32
Mass of oxygen = 33.28grams
Hence the mass of oxygen that reacted is 33.28 grams