In order to determine the initial termperature of the acetone, consider it as an idel gas. Then, you can write the following relation between volumes and temperatures:
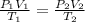
In this case, consider that the pressure is constant, then, P1=P2=P.
You cancel out pressure from the previous equation, solve for T1 and replace the values of the other parameters, as follow:
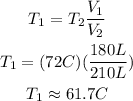
Hence, the initial temperature of the acetone was approximately 61.7°C