ANSWER
The final volume of the ballon is 2.0L
Explanation:
What to find? The final volume of the balloon
Given parameters
The initial pressure of the oxygen gas = 1.0 atm
The initial volume = 4.0 L
The final pressure of the gas = 2.0 atm
To find the final volume of the gas, we will need to apply Boyle's law
Boyle's law states that the volume of a given gas is inversely proportional to its pressure provided that the temperature remains constant.
This law can be expressed mathematically below
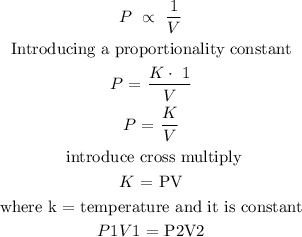
The next step is to substitute the parameters into the formula
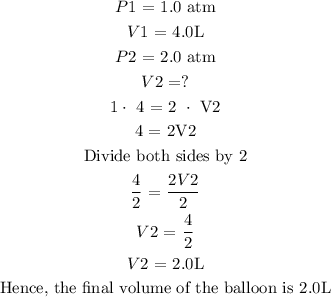
According to the above calculations, we observed that there is a decrease in the volume of the ballon
PART B
The gas law demonstrated is Boyle's law