The mole fraction of a solution is the number of moles of NaCl, the solute, divided by the total number of moles of solution:
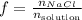
If we have 1 mol of solution, then:
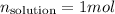
And the mole fraction is given, so:
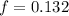
So, to calculate the number of moles of NaCl, we can solve for it and input the given values:
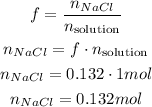
So, there are 0.132 moles of NaCl in the solution.