We know that a gas mixture contains 79% nitrogen and 21% oxygen. We must find the partial pressures if the total pressure is 1.02 atm.
In order to find the partial pressures we must use the next equation
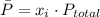
Where,
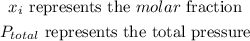
1. For nitrogen which is in a 79%
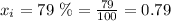
Now, replacing in the formula for the partial pressure
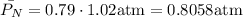
2. For oxygen which is in a 21%
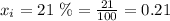
Now, replacing in the formula for the partial pressure
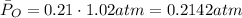
ANSWER:
- The partial pressure of the nitrogen is 0.8058 atm.
- The partial pressure of the oxygen is 0.2142 atm.