We are asked to determine the number of electrons in 1.5 Coulomb of charge. To do that we will use the following formula:
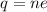
Where:
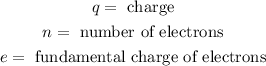
The fundamental charge is the charge of 1 electron and is given by:

Now, we substitute the values:
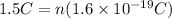
Now, we solve for "n":
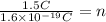
Solving the operations we get:
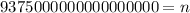
Therefore, there are 9375000000000000000 electrons.