For the equilibrium constant we have the following equation:
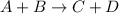
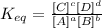
So, we have the products above and the equilibrium constant is directly proportional to the concentration of products. Therefore, if we want to obtain a higher concentration of products, we must look for the highest value of the constant.
In this case it will be 3.5, so the answer is c)Keq 3.5