Answer
The molecular formula of the sugar is C₈H₁₆O₈
Step-by-step explanation
The empirical formula is CH2O
The empirical molar mass = (1 x 12.011 g/mol C) + ( 2 x 1.008 g/mol H) + (1 x 15.999 g/mol O) = 30.026 g/mol
To get the molecular formula, we shall divide the molecular mass by the empirical mass and then multiply the answer with subscript of the empirical formula.
molecular mass = 240.0 g
empirical molar mass = 30.026 g/mol
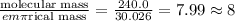
Molecular formula = (CH₂O)₈ = C₈H₁₆O₈
The molecular formula of the sugar is C₈H₁₆O₈