ANSWER
Q = 5303.95 kJ
Step-by-step explanation
We are looking for the energy needed to evaporate 2.35kg of water. Since the water is already at evaporation temperature, which is 100°C, we just have to use latent heat. Also, we just want the steam to be at 100°C, so we don't need to find the energy to change its temperature.
The energy needed to evaporate water of mass m is:
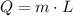
L is the latent heat of the material, in this case water. There are two latent heats, one of fusion and one of vaporization. We have to use the second one, which for water is approximately 2257 kJ/kg
Therefore, to boil 2.35 kg of water at 100°C to steam at 100°C we need:
![Q=2.35kg\cdot2257\frac{kJ}{\operatorname{kg}}=5303.95kJ]()