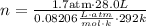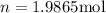We know that:
- Pressure of the oxygen gas = 1.7 atm
- Volume of the oxygen gas = 28.0 L
- Temperature of the oxygen gas = 292 k
In order to find the number of moles of has in the cylinder we must use the ideal gas equation

Where,
- P: Pressure
- V: Volume
- T: Temperature
- n: number of moles
- R: Universal gas constant
Since we must find the number of moles we need to solve the formula for n
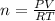
Finally, we must replace the values in the formula for n