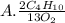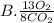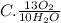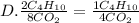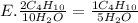They give us the balanced equation of the reaction. To know the ratios of each relationship we must see the coefficients that accompany the molecules, these will be the terms of the ratio. As follows:
2C4H10
13O2
8CO2
10H2O
Now we will replace these values according to the requested relation and simplify to the minimum possible expression: