Answer:
0.081atm
Explanations:
According to general gas equation expressed as:
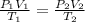
where:
• P1 and P2 are the ,initial and final pressure
,
• V1 and V2 are the ,initial and final volume
,
• T1 and T2 are the i,nitial and final temperature
Given the following parameters
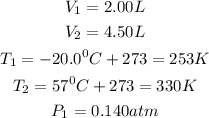
Required
Final Pressure P2
Substitute the given parameters into the formula
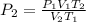
Substitute the given following parameters into the formula
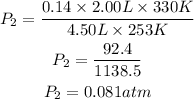
Hence the final pressure of the gas sample is 0.081atm