The first step is to write the combustion reaction of hexane:
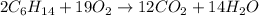
Now, we can use Avogadro's Number to find the amount of moles of hexane and O2 that are reacting:
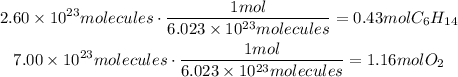
Using the stated reaction we can determine the amount of moles of O2 that react with 0.43 moles of hexane:
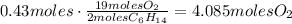
From this we can conclude that the limiting reactant is the O2, which means that we have to use the amount of O2 to make the calculations. Use this amount and the stoichiometric ratio of O2 and H2O to find the amount of water produced from 1.16moles of oxygen:
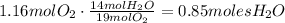
Now, we can use Avogadro's number to convert the amount of moles produced to molecules:
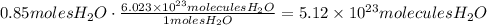
It means that 5.12x10^23 molecules of water are produced.