Answer:
276 days
Step-by-step explanation:
P.S - the exact question is -
To find - Determine the amount of time for polonium-210 to decay to one fourth its original quantity. The half-life of polonium-210 is 138 days. Please show all work.
Proof -
Given that , The half-life of polonium-210 is 138 days
⇒ Amount of polonium -210 to decay to
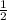 of its original quantity = 138 days
of its original quantity = 138 days
Now,
Amount of polonium to decay to next
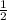 = 138 days
= 138 days
∴ we get
Total Amount of polonium -210 to decay to
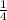 of its original quantity =138 + 138 days
of its original quantity =138 + 138 days
= 276 days
⇒Total amount of time for polonium-210 to decay to one fourth its original quantity = 276 days.