1) Balance the chemical equation
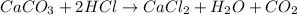
2) Convert grams to moles.
The molar mass of CaCO3 is 100.086 g/mol
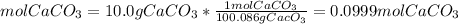
We have 0.0999 mol CaCO3.
The molar mass of HCl is 36.46 g/mol
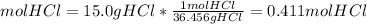
We have 0.411 mol HCl.
3) Which is the limiting reactant?
How many moles of CaCO3 do we need to use all of the HCl?
The molar ratio between CaCO3 and HCl is 1 mol CaCO3: 2 mol HCl.
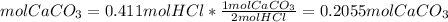
We need 0.2055mol CaCO3 and we have 0.0999mol CaCO3. We do not have enough CaCO3. This is the limiting reactant.
How many moles of HCl do we need to use all of the CaCO3?
The molar ratio between CaCO3 and HCl is 1 mol CaCO3: 2 mol HCl.
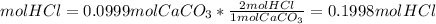
We need 0.1998mol HCl and we have 0.411 mol HCl. We have enough HCl. This is the excess reactant.
4) What mass of the excess reactant will get used?
We have 0.411 mol HCl
We need 0.1998 mol HCl. This is what we will use.
5) Convert moles to grams
The molar mass of HCl is 36.46 g/mol
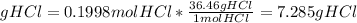
7.28g HCl will get used in the reaction.
.