Answer
14.6 g
Step-by-step explanation
Given:
Number of molecules of KI = 5.30 x 10²² molecules
What to find:
The grams of KI present in 5.30 x 10²² molecules.
Step-by-step solution:
Step 1: Convert the moles of KI to moles using Avogadro's number.
Conversion factor: 1 mole of any substance = 6.022 × 10²³ molecules
Therefore, the moles of KI in 5.30 x 10²² molecules will be
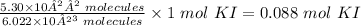
Step 2: Use the mole formula to determine the mass of Ki in 0.088 mol KI.
Note: The molar mass of KI = 166 g/mol
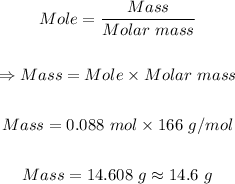
Therefore, 14.6 g of KI are in 5.30 x 10²² molecules of potassium iodide(Kl)