Explanation and answers:
In each case we have to identify the oxidation half-reaction and the reduction half-reaction, by using a standard reduction potencial table. Also, with the potencials of each helf-reaction we can calculate the voltage of each cell.
• Voltaic cell 1,:
In this case, the half-reaction of Mg is lower in the table of reduction potencials, therefore it is a strong reducing agent (it oxidizes), causing Zn to be reduced, that's why we must turn the helf reaction of Mg and its sign. The flow of electrons will go from the anode of Mg to the cathode of Zn, like we can see in the diagram.
- Oxidation half-reaction:
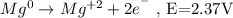
- Reduction half-reaction:
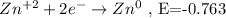
- Voltage of the cell:
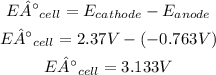
So, the voltage of the cell is 3.133V.
• Voltaic cell 2,:
In this case, the half-reaction of Mg is lower in the table of reduction potencials, therefore it is a strong reducing agent (it oxidizes), causing Cu to be reduced, that's why we must turn the helf-reaction of Mg and its sign. The flow of electrons will go from the anode of Mg to the cathode of Cu, like we can see in the diagram.
- Oxidation half-reaction:
![Mg^0\operatorname{\rightarrow}Mg^2+2e^-\text{, E}\operatorname{\degree}\text{=2.37V}]()
- Reduction half-reaction:
![Cu^(+2)+2e^-\operatorname{\rightarrow}Cu^0\text{, E}\operatorname{\degree}\text{=0.34V}]()
- Voltage of the cell:
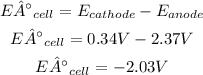
So, the voltage of the cell is -2.37V. The negative sign means that this cell is not spontaneous, the spontaneous reaction is the reverse reaction.