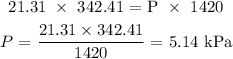Answer:
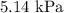
Explanations:
Here,we want to get the new pressure of the gas
To get this, we have to use the gas law that relates volume and pressure
This is the Boyle's law and it can be represented as follows:
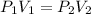
Where 1 represents the initial values and 2 represents the final values
We have to convert the volumes to the same scale
We have to convert the liters to ml by multiplying by 1000
We have this as 1.42 * 1000 = 1420 mL
The values are:
Initial
Pressure = 21.31 kPa
Volume = 342.41 mL
Final
Pressure =?
Volume = 1420 mL
We substitute the values as follows: