Answer: pH = 8.0
Step-by-step explanation:
The question requires us to calculate the pH of a solution which contains a hydrogen ion concentration of 1.0×10−8 M.
The pH of a solution can be calculated from the hydrogen ion concentration ([H+]) according to the following equation:
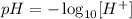
Thus, applying the hydrogen ion concentration provided by the question:
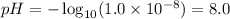
Therefore, the pH of the solution is 8.0.