ANSWER
The volume of water required to reduce the concentration is 713.34 mL
Explanation
Given information
The inital concentration of HCl = 0.27 M
The initial volume of HCl = 264.2 mL
The final concentration of HCl = 0.10 M
Let the volume of water be V2
To calculate the volume of water required to reduce the concentration of HCl, we will need to apply the dilution formula
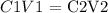

Therefore, the volume of water required to reduce the concentration is 713.34 mL