Answer
1.45 atm
Step-by-step explanation
Given:
The initial volume of the balloon, V₁ = 0.788 L
Initial pressure, P₁ = 1.20 atm
When some naughty child squeezes it down, the final volume, V₂ = 0.650 L
What to find:
The final pressure, P₂ when some naughty child squeezes the balloon down.
Step-by-step solution:
The final pressure, P₂ can be calculated using Boyle's law equation:
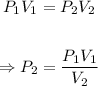
Plug in the values of the given parameters into the equation above:
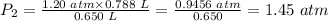
The pressure when some naughty child squeezes the balloon down = 1.45 atm.
The second option is the correct answer.