INFORMATION:
We have the next compound:
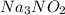
And we must find the oxidation state of the nitrogen.
STEP BY STEP EXPLANATION:
To find the oxidation state of an element, we need to use the next general rules
Now, using that in a neutral compound all oxidation numbers must add up to zero we have the next equation
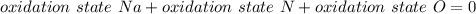
Then, since the Na is on the group one of the periodic table, Na has a plus one oxidation number and for Oxygen is -2. So,
Oxidation state Na = number of Na in the compound(oxidation number) = 3(+1) = +3
Oxidation state N = x (we don't know it yet)
Oxidation state O = number of O in the compound(oxidation number) = 2(-2) = -4
Finally, replacing in the equation and solving for x
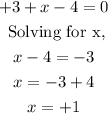
ANSWER:
The oxidation state of the nitrogen in Na3NO2 is +1