The given mass of water is,
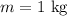
The value of the raise in the temperature is,
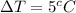
The value of the specific heat of the water is,
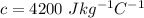
Thus, the energy needed to increase the temperature of water is,
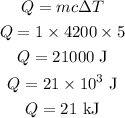
Hence, the amount of energy required to raise the temperature of the water to 5 degree C is 21 kJ.