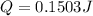We are asked to determine the amount of heat requiered to thaw a pacakge of vegetables. To do that we will use the following formula:
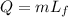
This is the formula of latent heat of fusion, where "Q" is the amount of heat, "m" is the mass, and Lf the heat of fusion. The heat of fusion is:
![L_f=0.334\frac{J}{\operatorname{kg}}]()
Which is the heat of fusion of water. Replacing the values:
![Q=(0.450\operatorname{kg})(0.334\frac{J}{\operatorname{kg}})]()
Solving the operations: