To find the slope of the given equation, we have to rewrite in slope intercept form. To do it, we have to solve the equation for y, it means, isolate it to one side of the equation, this way:
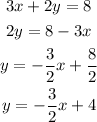
The slope of the line is the coefficient of x in the slope intercept form.
It means that the slope of the line is -3/2.