Chemistry => Chemical reactions => Balancing equations
We have a synthesis reaction, two compounds are linked to form a single compound. To balance the equation we must start by counting the atoms of each element on both sides of the reaction.
We start by balancing the sulfur. We have 8 sulfur atoms on the reactants. So we write coefficient 8 on the SO3 molecule:
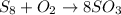
Now, we balance oxygen atoms. We have 24 oxygen atoms on the products. We put coefficient 12 on the O2 molecule, so we will have:
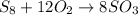
We have 8 sulfur atoms and 24 oxygen atoms on both sides of the reaction, so the equation is balanced now.
Answer: S8 + 12O2 → 8SO3