Answer and Explanation
Given the equation
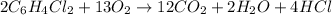
(a)
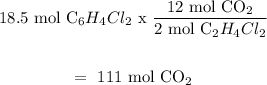
(b)
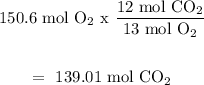
(c) C6H4Cl₂ is the limiting reactant because it will produce less amount of CO2 moles.
(d) The theoretical yield of CO2:
m = n x M where m is the mass (theoretical yield), n is the moles and M is the molar mass of CO2
m = 111 mol x 44,01 g/mol
m = 4885.11 g