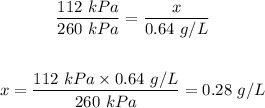Answer
Step-by-step explanation
Note that the basic relationship between the solubility and temperature can be given by: The higher the temperature is, the easier a solid will be able to dissolve. Likewise the lower is the temperature the harder is for a solid element to dissolve.
However, for a gas, the higher the temperature is, the more there is a decrease in the gas solubility. The lower the is temperature the higher is a gas solubility in water.
Therefore,