Answer
5.47 moles of oxygen gas are present in 175 g.
Step-by-step explanation
The number of moles of oxygen gas present in 175 g can be known by dividing the mass of the oxygen gas by its molar mass.
The molar mass of O₂ = 31.998 g/mol
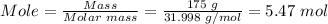
Therefore, 5.47 moles of oxygen gas are present in 175 g.